Efficient and Cost-effective Catalysis at the Single Atom Limit
Platinum and palladium are ubiquitous catalysts for the production of many chemicals and fuels as well as key components in clean energy technologies (1). However, their scarcity in nature and high price will limit their future proliferation. The Sykes lab has pioneered a new approach to designing heterogeneous catalysts that dilute these precious elements down to the single atom limit in much cheaper metals such as copper (2). These so-called Single Atom Alloys display high reaction selectivity and increased tolerance to carbon monoxide, a common catalyst poison (2-9).
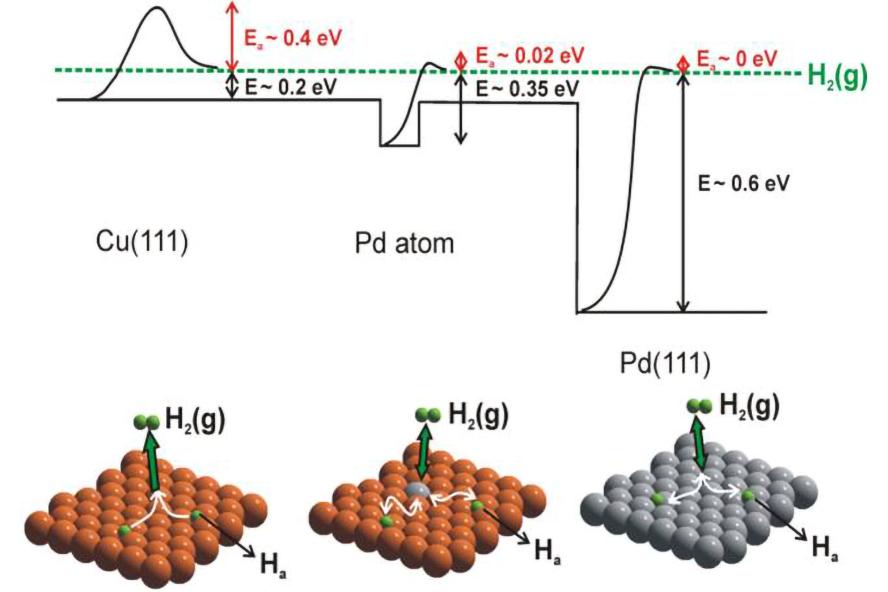
Potential energy landscape showing how single atom alloys offer completely different reaction kinetics and thermodynamics to the parent metals.
For example, we designed and tested a new generation of platinum and copper model catalysts for the selective hydrogenation of butadiene, an industrially important reaction (9). Butadiene poisons polymerization catalysts in industrial alkene streams used to produce approximately 42 million tons of polypropylene annually. The selective hydrogenation of butadiene to butene serves to increase the purity of the alkene feedstock without reducing its overall concentration. Therefore, catalysts that selectively hydrogenate butadiene to butene and prevent the further hydrogenation of butene are of great interest. We discovered that isolated platinum atom geometries enable hydrogen activation and spillover, but are incapable of carbon-carbon bond scission that leads to loss of selectivity and catalyst deactivation. Real nanoparticle catalysts with less than 1 platinum atom per 100 copper atoms exhibit high activity and selectivity for butadiene hydrogenation to butenes under mild conditions, demonstrating transferability from our model studies to the catalytic reaction under practical conditions (9).
Our single atom alloy approach may in fact prove to be a general strategy for designing novel bi-functional heterogeneous catalysts in which a catalytically active element is atomically dispersed in a more inert matrix (2). Moreover, some of the best industrial alloy catalysts to date may already be operating via this mechanism, but there is currently no method to directly probe the atomic geometry of a working catalyst. Our work is the first to definitively demonstrate that individual platinum and palladium atoms can facilitate efficient hydrogenation reactions (2-9). From a practical application standpoint, the small amounts of precious metal required to produce single atom alloys generate a very attractive alternative to traditional bimetallic catalysts.
- "Introduction to Surface Chemistry and Catalysis" G. A. Somorjai and Y. Li Wiley, 2nd Edition 2010, 1-800, ISBN 0-471-03192-5
- "Isolated Metal Atom Geometries as a Strategy for Selective Heterogeneous Hydrogenations" G. Kyriakou, M. B. Boucher, A. D. Jewell, E. A. Lewis, T. J. Lawton, A. E. Baber, H. L. Tierney, M. Flytzani-Stephanopoulos and E. C. H. Sykes - Science 2012, 335, 1209-1212.
- "Controlling the Spillover Pathway with the Molecular Cork Effect" M. D. Marcinkowski, A. D. Jewell, M. Stamatakis, M. B. Boucher, E. A. Lewis, C. J. Murphy, G. Kyriakou and E. C. H. Sykes - Nature Materials 2013, 12, 523-528.
- "Tackling CO Poisoning with Single Atom Alloy Catalysts" J. Liu, F. R. Lucci, M. Yang, S. Lee, M. D. Marcinkowski, A. J. Therrien, C. T. Williams, E. C. H. Sykes, and M. Flytzani-Stephanopoulos - Journal of the American Chemical Society 2016, 138, 6395-6399.
- "An Atomic-scale View of Palladium Alloys and their Ability to Dissociate Molecular Hydrogen" A. E. Baber, H. L. Tierney, T. J. Lawton and E. C. H. Sykes - ChemCatChem 2011, 3, 607-614.
- "Atomic-Scale Geometry and Electronic Structure of Catalytically Important Pd/Au Alloys" A. E. Baber, H. L. Tierney and E. C. H. Sykes - ACS Nano 2010, 14, 1637 - 1645.
- "Hydrogen Dissociation and Spillover on Individual Isolated Palladium Atoms" H. L. Tierney, A. E. Baber, J. R. Kitchin and E. C. H. Sykes - Physical Review Letters 2009, 103, 2461021-2461024.
- "Single Atom Alloy Surface Analogs in Pd0.18Cu15 Nanoparticles for Selective Hydrogenation Reactions" M. B. Boucher, B. Zugic, G. Cladaras, J. Kammert, M. D. Marcinkowski, T. J. Lawton, E. C. H. Sykes, and M. Flytzani-Stephanopolous - Physical Chemistry Chemical Physics 2013, 15, 12187-12196.
- "Selective Hydrogenation of 1,3-butadiene on Platinum-Copper Alloys at the Single-Atom Limit" F. R. Lucci, J. Liu, M. D. Marcinkowski, M. Yang, L. F. Allard, M. Flytzani-Stephanopoulos, and E. C. H. Sykes - Nature Communications 2015, 6, 8550.