Research
Complex carbohydrates play critical roles in a number of biological processes including, protein folding, cellular adhesion, signaling, and modulating the biological activity of natural products. Despite their importance, very little is understood about the molecular basis of their activity. Research in the Bennett lab is directed at developing new and efficient methods for carbohydrate synthesis, and the application of these methods to developing carbohydrate based therapeutics with novel modes of action. Representative projects include:
Reagent Controlled Methods for Stereoselective Carbohydrate Synthesis
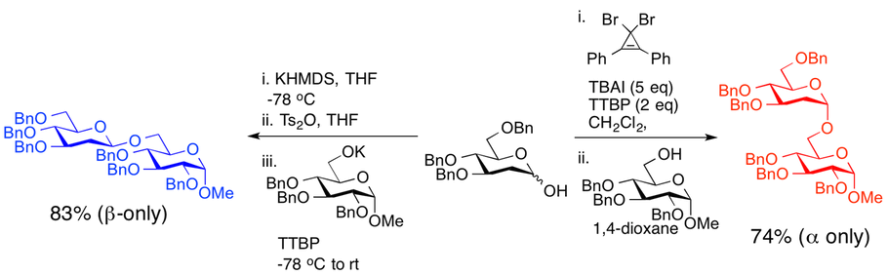
One of the biggest hurdles to carbohydrate synthesis lies in controlling the stereochemical outcome of glycosylation reactions. To address this issue, we are developing new chemical methodologies for the stereoselective (or stereospecific) construction of glycosidic linkages. Linkage starting from the same coupling partners, simply by changing the glycosylation promoter. We have demonstrated that this approach is effective in the construction of 2-deoxy-sugars and 1,2-cis-a-linked glycosides, two of the most difficult glycosidic linkages to synthesize using conventional approaches. This methodology has now been applied to the construction of several difficult to access deoxy sugar oligosaccharides. Ongoing projects include:
- examining the scope and mechanism or our approach
- the synthesis of complex oligosaccharides and small molecule glycoconjugates.
Total Synthesis of Antimicrobial Complex Carbohydrates
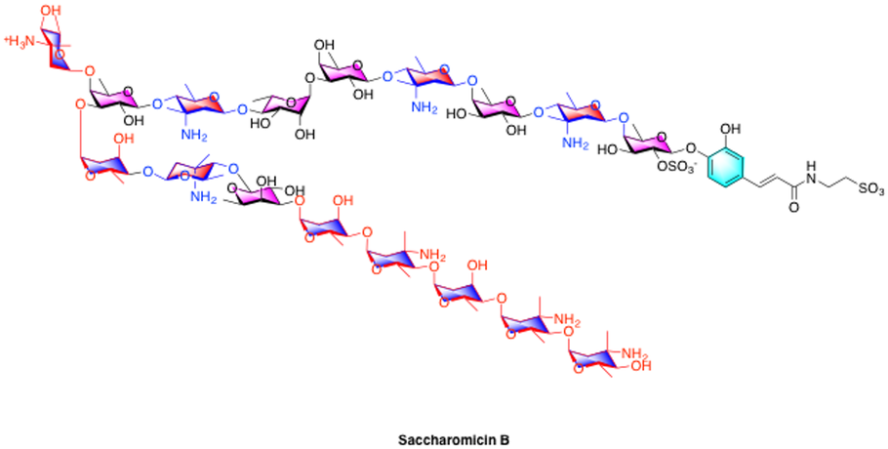
Increasing drug resistance in bacteria coupled with a paucity of new antibiotics is setting the stage for what might well be the greatest public health crisis of the 21st century. As a result, new therapies are necessary to combat drug resistant pathogens. One such compound is saccharomicin B. Isolated by Kong and coworkers, this molecule displayed potent activity against both gram positive and gram-negative pathogens. We are examining the application of our methodologies to the total synthesis of Saccharomicin B, with the long-term goal of establishing a route that is capable of supplying analogs of this potent natural product. Additional projects involve investigating the role glycosylation plays in bioactive small molecule glycoconjugates.
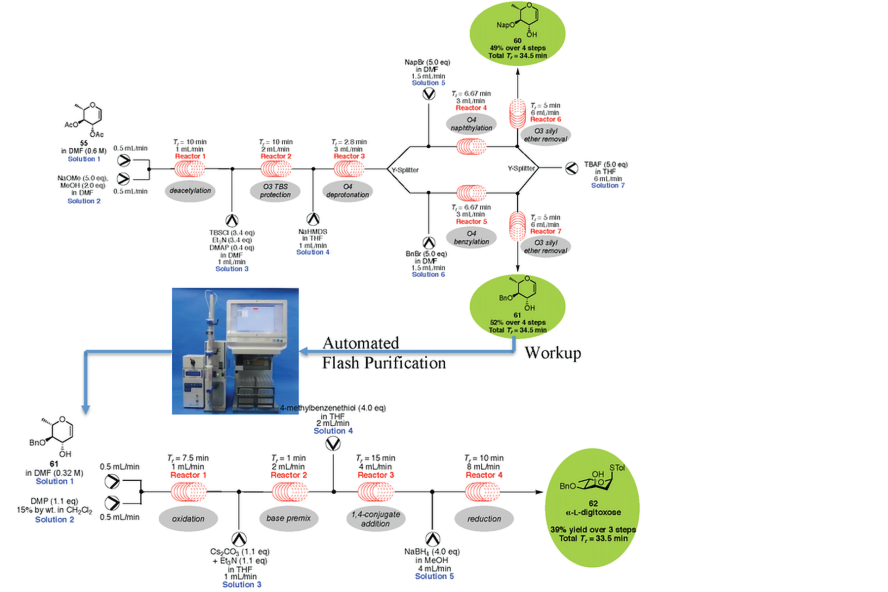
Automation of Carbohydrate Synthesis Using Continuous Flow Processing
The production of suitably protected monosaccharides ready for chemical glycosylation is arguable one of the most time consuming processes in oligosaccharide synthesis. This is especially true in the case of "unusual" sugars that are not commercially available, such as those from secondary metabolites and prokaryotes. We have demonstrated that a continuous flow platform permits the functionalization of monosaccharides on much faster time scales than is possible in conventional batch synthesis. By telescoping several reactions into a single sequence, this approach permits the construction of protected monosaccharides from commercially available precursors in mere hours. Furthermore, since many redox chemistries are amenable to continuous flow processing, this system permits the rapid synthesis of unusual sugars that otherwise require a week or more to construct in batch. This inexpensive system can be fully automated by interfacing it with the open source python based MechWolf program. We are currently extending this technology to fully automated oligosaccharide synthesis.